
In the rapidly advancing landscape of precision oncology, radiopharmaceuticals are driving a paradigm shift. This class of therapy is revolutionizing cancer care by merging the pinpoint accuracy of diagnostic imaging with the potent, targeted impact of radionuclide therapy.
Contents
- Harnessing the Power of Imaging in the Golden Age of Radiopharmaceutical Trials
- What are radiopharmaceuticals? The “See What You Treat” Revolution
- The Unique Demands of Radiopharmaceutical Trials
- Why is a Specialized Imaging Strategy Non-Negotiable for Radiopharmaceutical Clinical Trials?
- Navigating Complexity: What Sponsors and CROs Must Master in Radiopharmaceutical Trials
- The Future of Imaging in Radiopharma: New Targets, New Isotopes, New Hope
Harnessing the Power of Imaging in the Golden Age of Radiopharmaceutical Trials
In the rapidly advancing landscape of precision oncology, radiopharmaceuticals are driving a paradigm shift. This class of therapy is revolutionizing cancer care by merging the pinpoint accuracy of diagnostic imaging with the potent, targeted impact of radionuclide therapy. As the radiopharmaceutical therapy market is expanding rapidly — with projections showing up to 20% growth by 2033 [1], the necessity for deep imaging expertise and data-driven, actionable insights has never been more critical.
What are radiopharmaceuticals? The “See What You Treat” Revolution
Radiopharmaceuticals are smart drugs that deliver radiation directly to tumors by seeking out specific biological targets on cancer cells [2]. This mechanism embodies the principle of “see what you treat, treat what you see.”
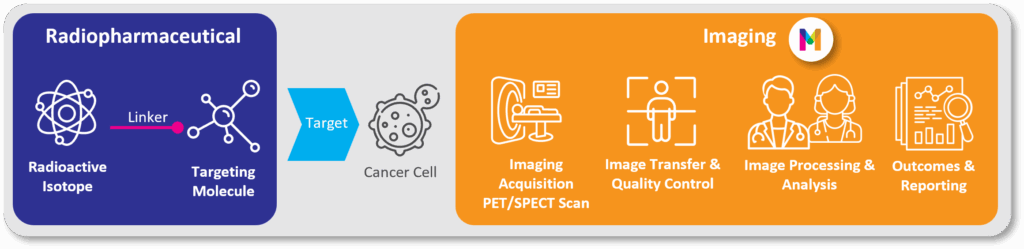
Image 1: Radiopharmaceuticals in Imaging for Clinical Trials: How Does it Work?
- Depending on the radioisotope used, these agents can serve two roles:
As diagnostic tools: Using isotopes like Gallium-68 or Fluorine-18, they function as tracers for PET/SPECT scans to precisely locate and characterize disease. - As therapeutic agents: Using isotopes like the beta-emitter Lutetium-177 or next-generation alpha-emitters like Actinium-225, they deliver cell-killing radiation directly to tumors.
This powerful dual function, known as theranostics, is the cornerstone of a new era in personalized cancer care. It enables physicians to select the right patients through imaging, deliver targeted treatment, and monitor therapeutic response —all within a single, efficient framework.
[1] The state of radiopharmaceutical therapy- Theranostics
[2] Radiopharmaceuticals Emerging as New Cancer Therapy – NCI
The Unique Demands of Radiopharmaceutical Trials
While built on general oncology principles, radiopharmaceutical trials are unique because imaging is not just a supporting tool—it is fundamental to the entire clinical process. Imaging is essential for:
- Verifying patient eligibility using imaging biomarkers.
- Visualizing drug biodistribution and quantifying radiation exposure to both tumors and healthy organs.
- Monitoring treatment response with specialized criteria like PERCIST, PCWG3 and PSMA
- Providing the robust, quantitative data needed for regulatory submissions, which is especially critical in trials with smaller patient populations.
Why is a Specialized Imaging Strategy Non-Negotiable for Radiopharmaceutical Clinical Trials?
A sophisticated and robust imaging strategy is directly linked to trial success. The unique properties of radiopharmaceuticals, such as the short half-life of isotopes, create complex logistical and regulatory challenges. Radiopharmaceutical trial success requires:
- Harmonized and Calibrated Protocols: Ensuring consistent and reproducible data across multiple sites with varying equipment is crucial. Scanner calibration is fundamental for generating the quantitative data needed for dosimetry and advanced analyses.
- Centralized Image Management: Rigorous quality control and blinded independent central reviews are necessary to minimize variability and bias in image interpretation.
- Advanced Quantitative Imaging and Dosimetry: Moving beyond simple visual assessments, radiopharmaceutical trials now rely on quantitative metrics and sophisticated dosimetry modeling to optimize safety and efficacy.
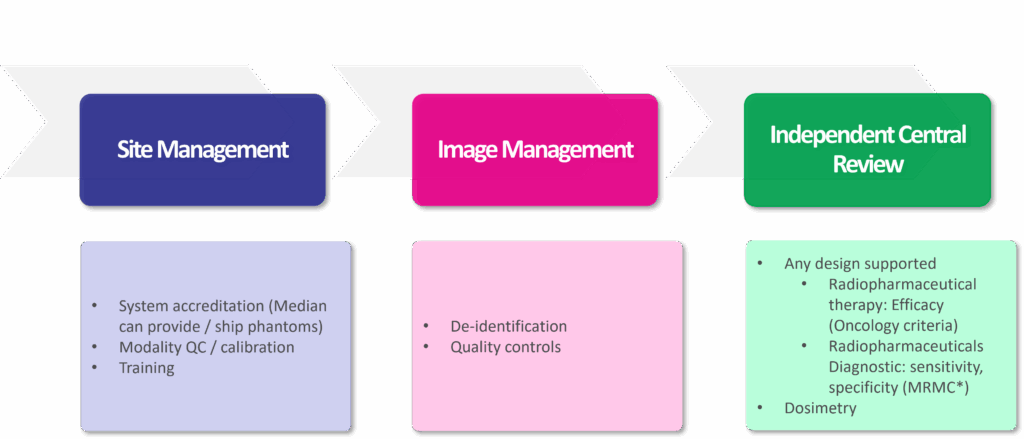
Image 2: Median’s Complete Solution for Radiopharmaceutical Image Processing
Navigating Complexity: What Sponsors and CROs Must Master in Radiopharmaceutical Trials
In the world of radiopharmaceutical development, success depends on the seamless integration of imaging at every stage. Because imaging underpins patient selection, treatment delivery, and response assessment, a highly specialized and coordinated strategy is paramount.
To navigate this intricate environment, sponsors must partner with an imaging CRO that possesses a deep understanding of nuclear medicine and offers a fully integrated, end-to-end solution. This includes:
- Early-Phase Expertise: Engaging nuclear medicine physicians, medical physicists, and radiopharmaceutical experts from the outset to design scientifically robust protocols aligned with the therapeutic mechanism.
- Defining Advanced Imaging Endpoints: Establishing endpoints that capture biological activity, biodistribution, and therapeutic efficacy while meeting evolving regulatory expectations for radiopharmaceuticals.
- Global Standardization and Training: Implementing standardized imaging protocols across all trial sites, including radiotracer handling, scanner calibration, and acquisition parameters, to ensure data integrity and regulatory compliance.
- Harnessing AI-Powered Analytics: Leveraging artificial intelligence and machine learning to accelerate and enhance image analysis. AI-driven tools can improve diagnostic accuracy, automate complex tasks like lesion segmentation, and support predictive dosimetry modeling, turning complex data into decisive insights.
- Integrated Scientific and Operational Oversight: Providing real-time image QC, continuous protocol optimization, and comprehensive support for regulatory documentation, including IND/CTA submissions and dosimetry reports.
By choosing an imaging CRO with this comprehensive, integrated approach, sponsors can ensure imaging is not just a technical task but a strategic asset that enhances the scientific rigor and regulatory success of their trials.
The Future of Imaging in Radiopharma: New Targets, New Isotopes, New Hope
The pipeline for radiopharmaceuticals is rapidly expanding. As therapies targeting PSMA, SSTR, and FAP mature, new agents aimed at targets like HER2, GRP, and DLL3 are entering clinical development. The future points toward:
- The Rise of Alpha-Emitters: Highly potent alpha particles like Actinium-225 are showing promise for treating resistant cancers.[3]
- Combination Therapies: Research is increasingly focused on combining radiopharmaceuticals with immunotherapy, chemotherapy, and other targeted agents to create synergistic effects.[4]
- Moving to Earlier Lines of Treatment: With proven efficacy and manageable side effects, there is a strong push to move radioligand therapies from late-stage to earlier-stage cancer treatment.[5]
Radiopharmaceuticals are transforming oncology, offering a highly personalized and effective approach to fighting cancer. However, without a meticulously planned and expertly executed imaging strategy, even the most promising therapies can falter in the clinic. Imaging is not merely for visualization—it is the bedrock of patient selection, treatment precision, response monitoring, and clinical validation. In radiopharmaceutical trials, imaging is not a supporting role—it is the foundation for success.
Want to know more about our radiopharmaceutical imaging solution? Contact us today.
[3] TRIUMF and actinium-225 – the ‘world’s rarest drug’
[4] Revolutionizing cancer treatment: The role of radiopharmaceuticals in modern cancer therapy – PMC
[5] Theranostic therapies expand frontline use in prostate cancer | Urology Times